• Ziwig Endotest®, how does it work?
MicroRNAs play an important role in vital biological pathways such as differentiation, proliferation and cell death, or the metastasis process. They are involved in the pathophysiology of many diseases (type 2 diabetes, cancers, cardiovascular diseases…). Numerous studies have shown that there is a direct link between the deregulation of certain microRNAs and the development of endometriosis lesions1,2.
Ziwig Endotest® uses two cutting-edge technologies, next-generation sequencing and artificial intelligence, to evaluate the entire human pool of microRNAs (approximately 2,600 microRNAs) for endometriosis from a simple saliva sample.
It is able to detect all types of endometriosis, from superficial to deep forms, including complex cases of “inconsistent/contradictory” patients (women with pain suggestive of endometriosis despite medical treatment to suppress menstruation but a normal clinical examination and radiological tests) with reliability close to 100% (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)³. It has been validated by the largest clinical trial ever conducted in this field3.
Women suffering from endometriosis, for the most part, go through a long medical journey (7 years on average), during which time they see multiple physicians and undergo numerous tests and examinations before a diagnosis, wich most often requires surgery, is finally reached.
The arrival of Ziwig Endotest® will in fact limit diagnostic delays and pave the way for early treatment of endometriosis, making it possible to slow down or even stop the aggravation of pain and other symptoms, optimize infertility care, and limit the deterioration of patients’ quality of life.
1- Panir K et al. Hum Reprod Update 2018;24(4):497-515.
2- Dana PM et al. Biomark Med 2020;14(13):1277-1287.
3- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https:/ doi.org/10.3390 jcm11030612
• What are microRNAs?
MicroRNAs are small non-coding RNAs: unlike messenger RNAs (nowadays used in several anti-COVID vaccines), they are not converted into proteins by the cellular machinery. On the contrary, their role is to supress in gene expression: when a microRNA binds to its target, a specific messenger RNA, it blocks or stimulates its transduction of proteins and/or induces its degradation.
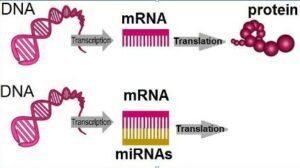 The regulation of gene expression by microRNAs (or miRNAs)
The regulation of gene expression by microRNAs (or miRNAs)
MicroRNAs are also present in the extracellular medium within different transport structures, that protect them from circulating RNases enzymes and gives them remarkable stability. These circulating miRNAs are found in varying amounts in most biological fluids (blood, urine, breast milk, tears, saliva, etc.)
MicroRNAs play an important role in vital biological pathways (such as differentiation, proliferation, and cell death, or the metastasis process) and as well as in the pathophysiology of many diseases (type 2 diabetes, cancers, cardiovascular diseases, etc.).
In recent years, increasing evidence has supported the involvement of miRNAs in the cellular mechanisms of endometriosis. A direct link between the deregulation of certain miRNAs and the development of endometriosis lesions has been established2,3.
The Ziwig Endotest® saliva test uses two cutting-edge technologies, next-generation sequencing and artificial intelligence, to evaluate the entire human microRNA pool (2561 microRNAs) for endometriosis from a simple saliva sample.
Its reliability is close to 100% (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)3.
1- Panir K et al. Hum Reprod Update 2018;24(4):497-515.
2- Dana PM et al. Biomark Med 2020;14(13):1277-1287.
3- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
• How do I get Ziwig Endotest®? How much does it cost? Is it reimbursed?
Ziwig Endotest®, which has CE rating in accordance with the European regulatory framework, will soon be available in several European countries.
In France, its availability to patients and its price are currently being evaluated by the health authorities, with a view to its inclusion in the care plan and its possible reimbursement by the health insurance system.
• What does Ziwig Endotest® offer compared to the existing means of diagnosing endometriosis?
The most commonly used examinations are pelvic ultrasound and pelvic MRI. Their diagnostic performance is sufficient for certain forms of endometriosis (ovarian cysts, deep endometriosis) but insufficient for others (peritoneal endometriosis).
Laparoscopy, a surgical procedure that must be carried out under general anesthesia, may in certain cases be necessary to search for peritoneal endometriosis.
The performance of the Ziwig Endotest® saliva test (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)³ proved to be superior to all other diagnostic exams1-5, whether medical imaging (pelvic MRI and ultrasound) or laparoscopy. Ziwig Endotest® also makes it possible to avoid unnecessary surgical interventions, espacially for patients with symptoms similar to endometriosis but who are not carriers of the disease.
Ziwig Endotest® thus represents a major advance in the diagnosis of endometriosis compared to existing tests.
Ziwig Endotest® is not intended to replace pelvic imaging (MRI, ultrasound). Medical imaging remains essential, after diagnosis, for the description of lesions, their mapping, their classification and for defining the prognosis of the disease.
1- Machine learning algorithms as new screening approach for patients with endometriosis.
Bendifallah S & al. Sci Rep. 2022 Jan 12;12(1):639. doi: 10.1038/s41598-021-04637-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8755739/pdf/41598_2021_Article_4637.pdf
2 – Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression.
Dabi Y & al. Diagnostics (Basel). 2022 Jan 12;12(1):175. doi: 10.3390/diagnostics12010175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774370/pdf/diagnostics-12-00175.pdf
3 – Salivary MicroRNA Signature for Diagnosis of Endometriosis.
Bendifallah & al. J Clin Med. 2022 Jan 26;11(3):612. doi: 10.3390/jcm11030612.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8836532/pdf/jcm-11-00612.pdf
4 – MicroRNome analysis generates a blood-based signature for endometriosis.
Bendifallah S & al. Sci Rep. 2022 Mar 8;12(1):4051. doi: 10.1038/s41598-022-07771-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902281/pdf/41598_2022_Article_7771.pdf
5 – Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study
Bendifallah S & al. Diagnostics. 2022; 12(5):1150.
https://doi.org/10.3390/diagnostics12051150
• Will Ziwig Endotest® replace MRI and ultrasound?
The Ziwig Endotest® saliva test offers healthcare professionals dealing with a patient presenting signs of endometriosis the possibility of rapid, reliable and non-invasive information to establish the diagnosis. It is equally effective in the diagnosis of early stages, complex forms and
advanced stages of the disease1-5.
Ziwig Endotest® is not intended to replace pelvic imaging (MRI, ultrasound). The two types of examination are complementary. After diagnosis, medical imaging remains indispensable for describing lesions, mapping and classifying them, and for defining the prognosis of the disease.
1- Machine learning algorithms as new screening approach for patients with endometriosis.
Bendifallah S & al. Sci Rep. 2022 Jan 12;12(1):639. doi: 10.1038/s41598-021-04637-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8755739/pdf/41598_2021_Article_4637.pdf
2 – Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression.
Dabi Y & al. Diagnostics (Basel). 2022 Jan 12;12(1):175. doi: 10.3390/diagnostics12010175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774370/pdf/diagnostics-12-00175.pdf
3 – Salivary MicroRNA Signature for Diagnosis of Endometriosis.
Bendifallah & al. J Clin Med. 2022 Jan 26;11(3):612. doi: 10.3390/jcm11030612.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8836532/pdf/jcm-11-00612.pdf
4 – MicroRNome analysis generates a blood-based signature for endometriosis.
Bendifallah S & al. Sci Rep. 2022 Mar 8;12(1):4051. doi: 10.1038/s41598-022-07771-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902281/pdf/41598_2022_Article_7771.pdf
5 – Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study
Bendifallah S & al. Diagnostics. 2022; 12(5):1150.
https://doi.org/10.3390/diagnostics12051150
• Can Ziwig Endotest® be used on adolescent girls?
The study that allowed the validation of Ziwig Endotest® was carried out in a population of women aged 18 to 43 years with chronic pain suggesting endometriosis¹. Ziwig Endotest® has not yet been evaluated in adolescents.
1- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
• Can Ziwig Endotest® diagnose all forms of endometriosis?
Ziwig Endotest® can detect superficial forms and deep forms of endometriosis, and it is an efficient diagnostic tool for complex cases among patients with pain suggestive of endometriosis but with a negative clinical examination and medical imaging, with a reliability close to 100% (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)1.
1- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
• How can a saliva test detect a disease that comes from the uterus?
Several studies have shown a direct link between the development of endometriosis lesions and the deregulation, at the cellular level, of small non-coding RNAs called microRNAs. These microRNAs play an important role in vital biological pathways such as differentiation, proliferation and cell death, as well as the metastasis process. They are involved in the pathophysiology of many diseases (type 2 diabetes, cancers, cardiovascular diseases…), and particularly in the pathophysiology of endometriosis. They are found in variable quantities in most biological fluids (blood, urine, breast milk, tears, etc.) including saliva, where they are present in high concentrations.
The prospective clinical trial Endo-miRNA⁴, evaluated salivary samples from 200 women with pain suggestive of endometriosis. It explored the entire human pool of microRNAs (2561 microRNAs) for each sample. Thanks to the use of two cutting-edge technologies, next-generation sequencing and artificial intelligence, all of these microRNAs were identified, analyzed, mapped and then quantified with regard to their possible link with endometriosis, allowing the development of a diagnostic test for endometriosis, Ziwig Endotest®, based on 109 microRNAs.
The performance of Ziwig Endotest® (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)³ is superior to that of all the diagnostic tools currently available (notably pelvic MRI and ultrasound)1-5.
1- Machine learning algorithms as new screening approach for patients with endometriosis.
Bendifallah S & al. Sci Rep. 2022 Jan 12;12(1):639. doi: 10.1038/s41598-021-04637-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8755739/pdf/41598_2021_Article_4637.pdf
2 – Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression.
Dabi Y & al. Diagnostics (Basel). 2022 Jan 12;12(1):175. doi: 10.3390/diagnostics12010175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774370/pdf/diagnostics-12-00175.pdf
3 – Salivary MicroRNA Signature for Diagnosis of Endometriosis.
Bendifallah & al. J Clin Med. 2022 Jan 26;11(3):612. doi: 10.3390/jcm11030612.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8836532/pdf/jcm-11-00612.pdf
4 – MicroRNome analysis generates a blood-based signature for endometriosis.
Bendifallah S & al. Sci Rep. 2022 Mar 8;12(1):4051. doi: 10.1038/s41598-022-07771-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902281/pdf/41598_2022_Article_7771.pdf
5 – Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study
Bendifallah S & al. Diagnostics. 2022; 12(5):1150.
https://doi.org/10.3390/diagnostics12051150
• To diagnose endometriosis, wouldn't a blood test be more reliable than a saliva test?
The Endo-miRNA prospective clinical trial analyzed the human microRNAome in order to distinguish patients with endometriosis from non-affected patientes, and to develop a diagnostic test based on blood and saliva microRNAs.
109 miRNAs from more than 2600 human miRNAs involved in the pathophysiology of endometriosis have been identified in saliva, and 86 in blood, providing a reliable signature of the disease1.
The performance of this “signature” for the diagnosis of endometriosis was almost the same in saliva and blood: sensitivity of 96.7%, specificity of 100%, diagnostic accuracy [AUC] of 98.3% in saliva; sensitivity of 96.8%, specificity of 100%, AUC of 98.4% in blood. These results demonstrate the robustness and reproducibility of the method used1.
Saliva offers a better concentration of microRNA and better stability conditions than blood. Moreover, saliva sampling is non-invasive, whereas blood sampling requires complex storage and transportation logistics.
The idea of a salivary diagnostic test for endometriosis therefore naturally prevailed over that of a blood test.
1- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
• The study that validated Ziwig Endotest® was conducted on 200 patients. Is this enough?
Several preliminary studies involving less than 100 patients have, in recent years, demonstrated the value of microRNAs in the diagnosis of endometriosis.
However, due to methodological limitations (limited number of patients, lack of inclusion of all forms of endometriosis) and technical limitations (limited quantity of microRNAs evaluated without next-generation sequencing), none of these studies has led to the development of a sufficiently efficient diagnostic test for endometriosis in blood and even less so in saliva1,2,3.
The Endo-miRNA4 study stood out from previous studies by its holistic approach, analysing the entire human microRNA pool (the microRNAome), i.e. 2600 microRNAs, for each patient.
This approach was made possible by the use of 2 cutting-edge technologies:
– Next-generation sequencing, which allows for the simultaneous acquisition of data relating to millions of RNA fragments for each sample
– Artificial intelligence, combined with machine learning, which has made it possible to analyze and exploit the very large volume of data generated by next-generation sequencing and to generate a diagnostic signature for endometriosis with a reliability close to 100% (sensitivity of 97%, specificity of 100%, diagnostic accuracy (AUC) of 98%)⁴.
The Endo-miRNA study is the largest prospective trial of any medical specialty, in terms of the number of patients, the number of microRNAs and the number of samples taken (n=200 for blood and n=200 for saliva), to evaluate a diagnostic test for endometriosis based on microRNAs.
This study has been published in several peer-reviewed international scientific journals4,5.
1- Panir K et al. Hum Reprod Update 2018;24(4):497-515.
2- Dana PM et al. Biomark Med 2020;14(13):1277-1287.
3- Moustafa S et al. Am J Obstet Gynecol 2020;223(4):557.e1-557.e11
4- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
5- Dabi Y. et al. Diagnostics (Basel) 2022 ; 12(1):175. https://doi.org/10.3390/diagnostics12010175
• How reliable is Ziwig Endotest®?
Ziwig Endotest® has a sensitivity of 96.7%, a specificity of 100%, and a diagnostic accuracy (AUC) of 98.3%1.
These diagnostic performances are superior to all currently available diagnostic tools (including pelvic MRI and ultrasound).
1- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390 jcm11030612
• Can Ziwig Endotest® be wrong?
The sensitivity of a diagnostic test refers to the percentage of sick people who will be identified as such by the test (sick people who test positive).
The specificity of a diagnostic test refers to the percentage of people who are NOT sick who will be identified as such by the test (people who are NOT sick and whose test will be negative).
Given its high diagnostic performance (sensitivity of 96.7%, specificity of 100%)³ and the prevalence of endometriosis in the population (approximately 10%), Ziwig Endotest® results are very reliable1-5.
Nevertheless, it is impossible to exclude the existence of rare cases of erroneous results, whether they are false negatives (patients with a negative test when they are sick) or false positives (patients with a positive test when they are NOT sick).
1- Machine learning algorithms as new screening approach for patients with endometriosis.
Bendifallah S & al. Sci Rep. 2022 Jan 12;12(1):639. doi: 10.1038/s41598-021-04637-2.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8755739/pdf/41598_2021_Article_4637.pdf
2 – Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression.
Dabi Y & al. Diagnostics (Basel). 2022 Jan 12;12(1):175. doi: 10.3390/diagnostics12010175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774370/pdf/diagnostics-12-00175.pdf
3 – Salivary MicroRNA Signature for Diagnosis of Endometriosis.
Bendifallah & al. J Clin Med. 2022 Jan 26;11(3):612. doi: 10.3390/jcm11030612.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8836532/pdf/jcm-11-00612.pdf
4 – MicroRNome analysis generates a blood-based signature for endometriosis.
Bendifallah S & al. Sci Rep. 2022 Mar 8;12(1):4051. doi: 10.1038/s41598-022-07771-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902281/pdf/41598_2022_Article_7771.pdf
5 – Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study
Bendifallah S & al. Diagnostics. 2022; 12(5):1150.
https://doi.org/10.3390/diagnostics12051150
• For which women is Ziwig Endotest® intended ?
Ziwig Endotest® is an in vitro diagnostic device reserved exclusively for professional use for the diagnosis of endometriosis using a saliva sample.
Ziwig Endotest® is intended for patients aged 18 to 43 years with signs suggestive of endometriosis as part of a care pathway. The symptoms that may lead to the test being performed are:
-chronic pelvic pain
-dysmenorrhea
-abdominal pain out of period
-pain suggestive of sciatica
-lumbar pain out of period
-pain in the right shoulder during period
-blood in the stool during period
-blood in the urine during period.
If you are a healthcare professional and would like to be notified when the test is available, you can enter your email address here:
• When will Ziwig Endotest® be available?
Ziwig Endotest®, which has CE rating in accordance with the European regulatory framework, will soon be available in several European countries.
If you are a healthcare professional and would like to be notified when the test is available, you can enter your email address here:
• I would like to have this test immediately, is this possible?
Ziwig Endotest®, which has CE rating in accordance with the European regulatory framework, will soon be available in several European countries.
In France, its availability to patients and its price are currently being evaluated by the health authorities, with a view to its inclusion in the care plan and its possible reimbursement by the health insurance system.
If you are a healthcare professional and would like to be notified when the test is available, you can enter your email address here:
• Has Ziwig Endotest® been scientifically validated?
Ziwig Endotest®, is validated by the Endo-miRNA¹ trial, the largest clinical trial evaluating the value of microRNAs for the diagnosis of endometriosis.
This study has resulted in several articles published in international peer-reviewed scientific journals.
1- Bendifallah S. et al. J Clin Med 2022 ; 11(3):612. https://doi.org/10.3390/jcm11030612
Find the scientific studies here.
• Does Ziwig Endotest® have a rating?
Ziwig Endotest® has a CE rating, according to the current regulations (Medical Device In Vitro DM DIV CE Directive 98/75 outside Annex II).
In addition, ZIWIG complies with ISO 13485 – quality management system requirements for the medical device industry.
• In which countries is Ziwig Endotest® available?
Ziwig Endotest® will soon be available in Europe, North America and the Middle East.
• Will the technology used for Ziwig Endotest® be applied to other pathologies?
Yes, several clinical studies have been launched.

