Ziwig Endotest®,
the first salivary diagnostic test for endometriosis
reducing the diagnosis time to just a few days1.
Ziwig puts its technology and unique know-how at the service of women’s health and endometriosis in particular.
Ziwig Endotest® makes it possible to diagnose endometriosis at the patient’s location using a simple saliva sample.
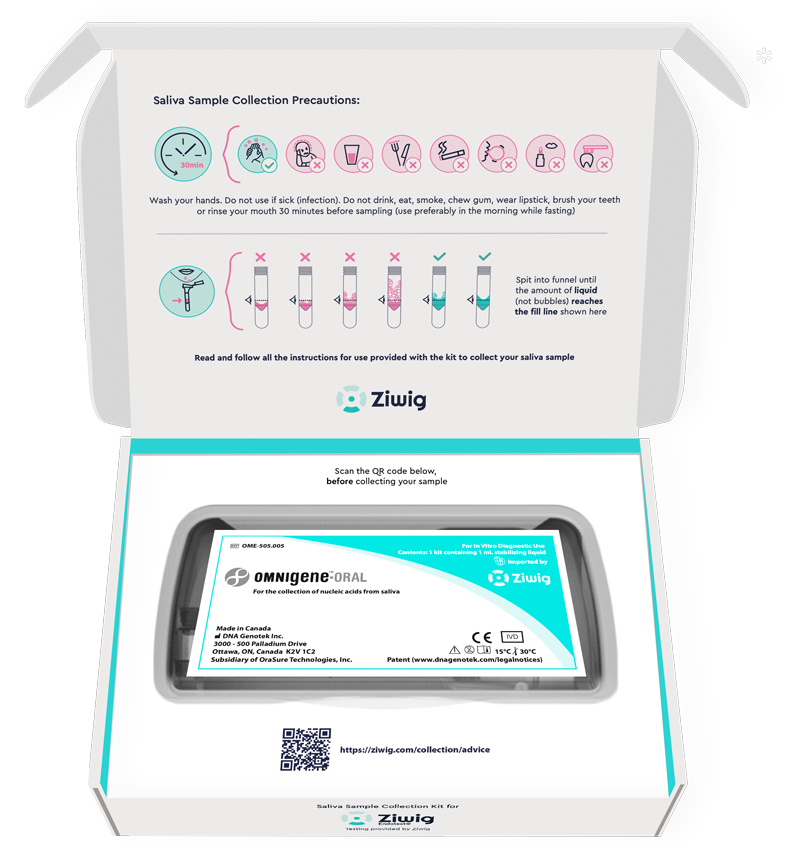

The centralised laboratory sequences salivary RNA
and the digital platform uses machine learning algorithms to deliver a reliable result using a proprietary and scientifically validated technique2.

Ziwig Endotest® is available on prescription for specific indications in a certain number of countries.
Ziwig Endotest® is an in vitro diagnostic device reserved exclusively for professional use for the diagnosis of endometriosis on salivary samples. Ziwig Endotest® is an innovative diagnostic method based on the analysis of salivary miRNAs and the identification of phenotypic profiles characteristic of endometriosis identified by NGS (Next Generation Sequencing) method and modeled by AI. Read carefully the information provided with Ziwig Endotest®. Manufacturer: Ziwig. Update: 10/2023.
1 – IFUED100v02
2- Bendifallah, Sofiane et al. “Validation of a Salivary miRNA Signature of Endometriosis — Interim Data” NEJM Evidence. 09 June. 2023. doi: 10.1056/EVIDoa2200282
*Omnigene Oral is an in vitro diagnostic device for the collection and stabilisation of nucleic acids in saliva. Manufacturer of the Omnigene Oral collection IVDD: DNA Genotek Inc. Read the enclosed leaflet carefully. Update: 10/2023.
Stay connected
Sign up to be notified of the latest news and publications.


