A saliva test for endometriosis providing a reliable diagnosis within just a few days

Where to find
Ziwig Endotest® ?
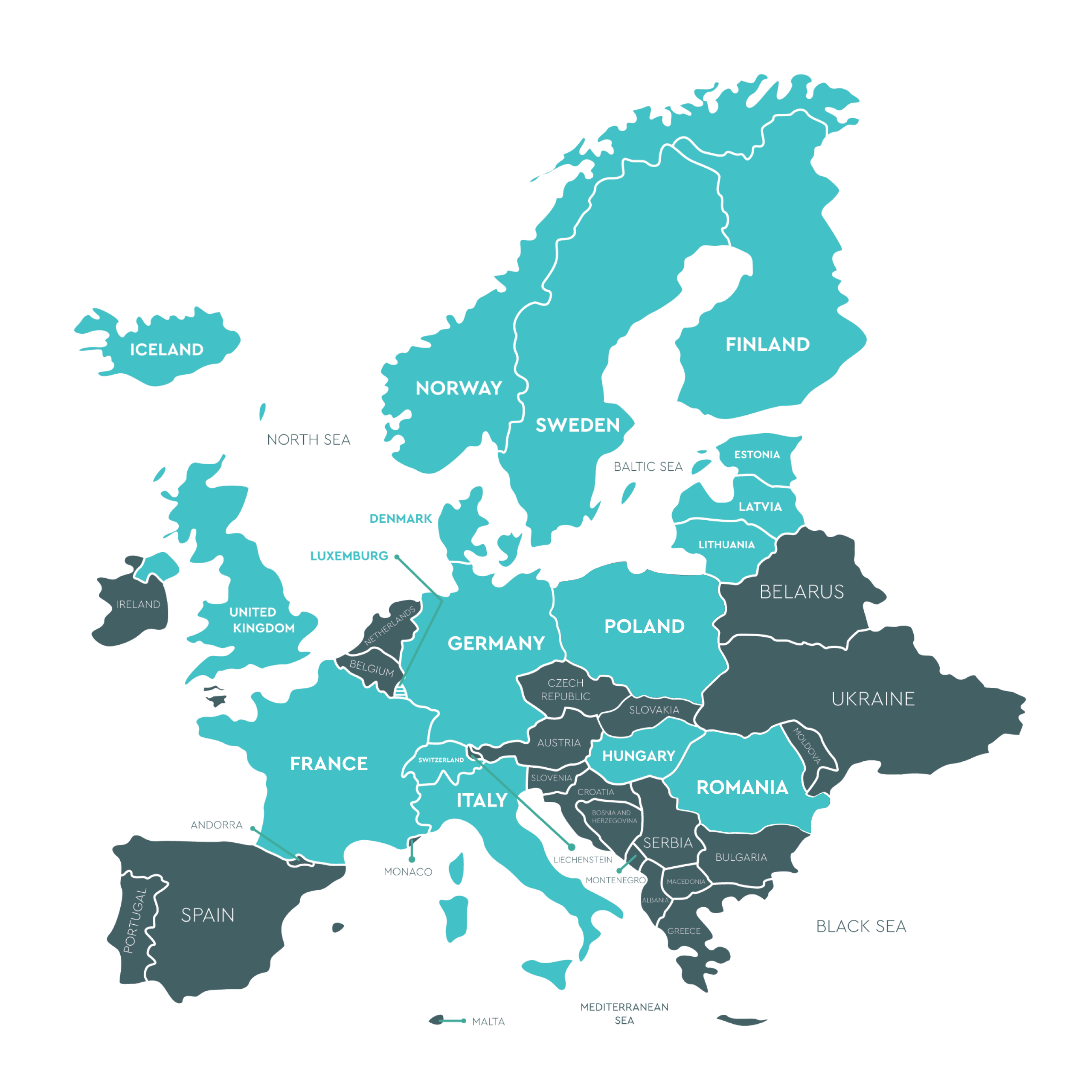
Europe
1
2
3
4
5
6
7
8
9
10
11
13
 Roumanie (Regina Maria)Mrs Pavelina Cristina Manea & Catalina Cristea – +40740200090 /+40731930541 laborator.genetica@reginamaria.ro
Roumanie (Regina Maria)Mrs Pavelina Cristina Manea & Catalina Cristea – +40740200090 /+40731930541 laborator.genetica@reginamaria.ro 14
Disponible en accès précoce
17
 Pologne (Instytut Diagnostyki Molekularnej DIAGMOL) Mrs Mila Ganc – +48 571994566 milaganc@diagmol.pl
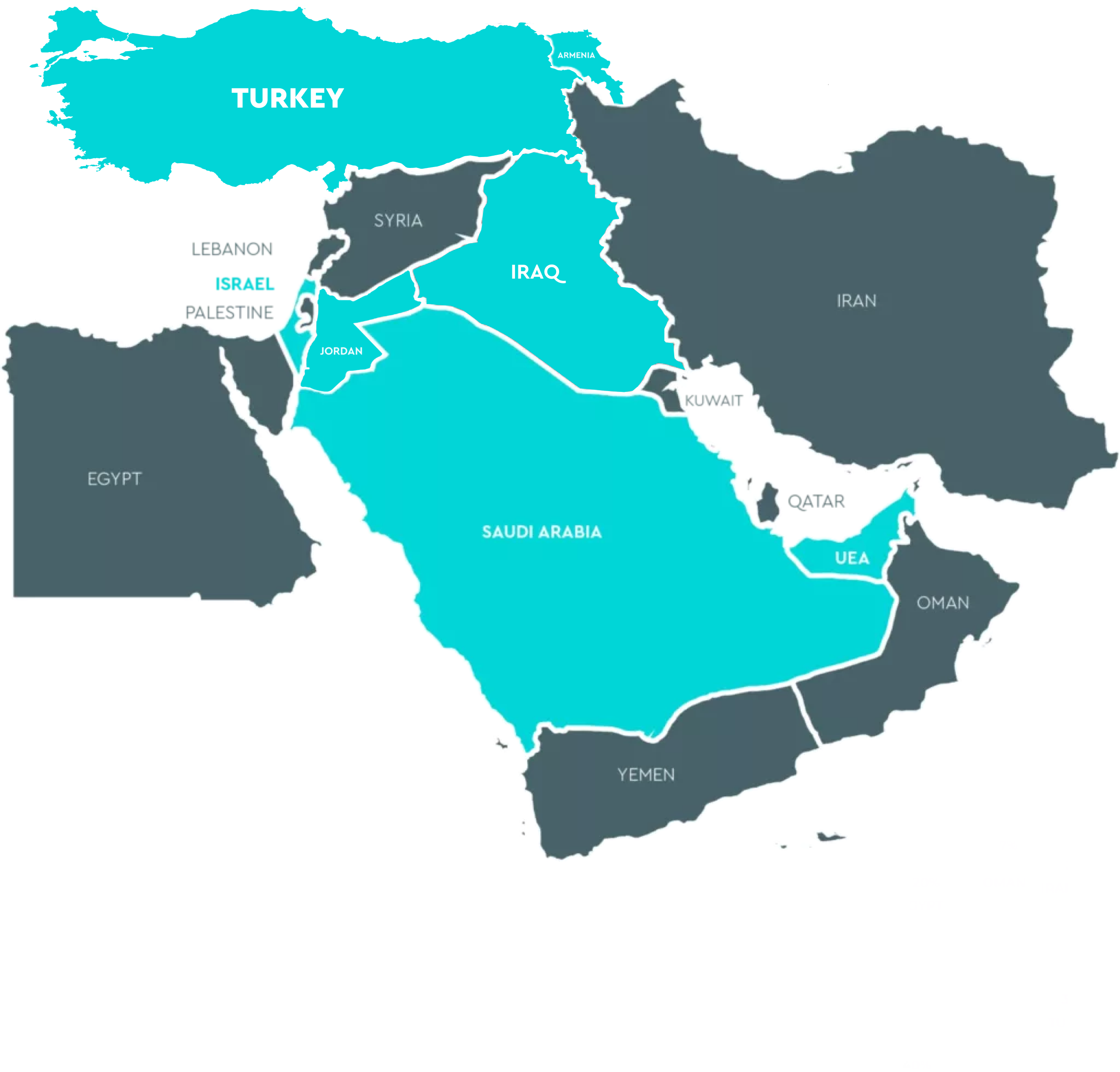
Pologne (Instytut Diagnostyki Molekularnej DIAGMOL) Mrs Mila Ganc – +48 571994566 milaganc@diagmol.pl Middle East
1
 United Arab Emirates (Cureleads) Mr Hussein Rammal – +971585700316 info@cureleads.com hussein.rammal@cureleads.com
United Arab Emirates (Cureleads) Mr Hussein Rammal – +971585700316 info@cureleads.com hussein.rammal@cureleads.com 2
Saudi Arabia (Al Habib Medical Group)
3
5
6
Irak (Warba)
7
 Turkey (Reprotek Medikal Danismanlik A.S) Mrs Didem Kilic Demircan – 0090312 807 2135 reprotek@reprotek.com.tr
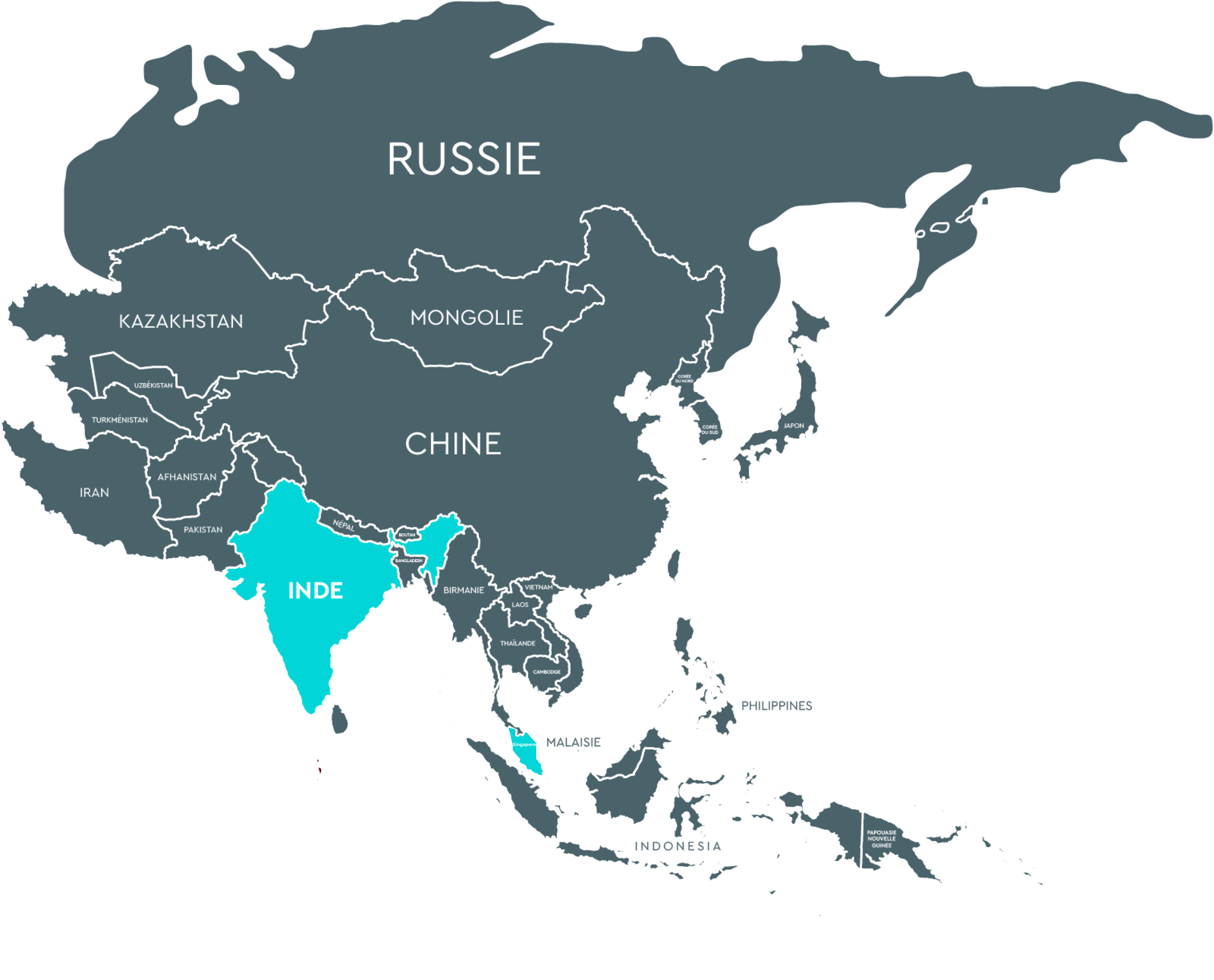
Turkey (Reprotek Medikal Danismanlik A.S) Mrs Didem Kilic Demircan – 0090312 807 2135 reprotek@reprotek.com.trASIA
1
 India (Precision woman’s care and research institute Pvt Ltd) Mr Tanuj Satish – +91 6282588315 tanujsatish@gmail.com
India (Precision woman’s care and research institute Pvt Ltd) Mr Tanuj Satish – +91 6282588315 tanujsatish@gmail.com2
Singapore (Compai Pharma)
Americas
1
Brazil (College Pesquisa Clínica) Mr Luiz Fernando Pina- +5511953436551 endotest.br@babycenter.med.br
2
Together, let’s usher in a new era in the diagnosis of endometriosis, marked by scientific innovation and precision, to improve patient care.

I am interested in
Ziwig Endotest®
Omnigene Oral is an in vitro diagnostic device for the collection and stabilisation of nucleic acids in saliva. Manufacturer of the Omnigene Oral collection IVDD: DNA Genotek Inc. Read the enclosed leaflet carefully. Updated: 09/2024
- Ziwig Endotest® is an in vitro diagnostic device for professional use in the diagnosis of endometriosis using a saliva sample.
- Ziwig Endotest® is an innovative diagnostic method using salivary miRNA analysis to identify phenotypic profiles characteristic of endometriosis identified by NGS (next-generation sequencing) and modelled by AI.
- This in vitro diagnostic medical device is a regulated health product which, in accordance with these regulations, bears the CE mark.
Please read the information supplied with Ziwig Endotest® carefully. Manufacturer: Ziwig. Last update : 09/2024 - Ziwig Endotest® has obtained CE certification from a notified body (2797).
Stay connected
Sign up to be notified of the latest news and publications.